1 AND ANATOMY EMBRYOLOGY, HISTOLOGY, · 2020-01-19 · 3 Embryology, Histology, and Anatomy Figure...
Transcript of 1 AND ANATOMY EMBRYOLOGY, HISTOLOGY, · 2020-01-19 · 3 Embryology, Histology, and Anatomy Figure...

1
EMBRYOLOGY, HISTOLOGY, AND ANATOMY1
EMBRYOLOGY
At the beginning of fetal development (to-ward the latter part of the third week of gesta-tion), the hepatic diverticulum buds from the ventral foregut. The hepatic diverticulum then gives rise to the transverse septum (septum transversum), a structure situated between the pericardial and peritoneal cavities (1,2). Mesenchymal elements of the transverse sep-tum, which already exist, are invested by liver parenchyma formed from hepatic endodermal cells (also known as hepatoblasts) of the he-patic diverticulum to give rise to the liver buds (3). During fetal development the liver buds function as a hematopoietic organ, which is composed of hepatocytic cords, venous-sinusoi-dal plexus, and hematopoietic precursor cells, including Kupffer cells (macrophages residing in liver). The umbilical vein supplies most of the blood during development of the liver. The remaining smaller amount of blood is supplied by the portal vein and hepatic artery.
During fetal development, the placenta ex-ecutes the major functions performed by the adult liver, including the absorption of nutri-ents and the excretion of bile containing waste products; bile secretion by fetal liver is negligible before birth. Nonetheless, the fetal liver produc-es alpha-fetoprotein and other plasma proteins, synthesizes bile acids, and stores fat, glycogen, iron, and copper.
The anterior portion of the hepatic diver-ticulum forms the liver and intrahepatic bile ducts, while the posterior portion gives rise to the gallbladder and extrahepatic bile ducts (2,4–6). The ultimate formation of intrahepatic bile ducts occurs late during development, and is completed after birth, during the first year of life. The development of small intrahepatic bile ducts begins when the ductal plate is formed, which is a single-layered sheath of small flat epithelial cells assembled by the periportal hepatoblasts in
intimate contact with the portal mesenchyme surrounding a portal vein branch (2). In the weeks that follow, certain parts of the ductal plates are duplicated by a second layer of cells, to become double-layered ductal plates, which then dilate to form cylindrical structures to be integrated into the mesenchyme of the newly formed portal region (fig. 1-1). When they are integrated into the portal space, the immature tubules evolve into bile ducts that are embraced by connective tissue and gradually situated in their usual location in the portal tracts as the portal tracts increase in size (2,7–9).
GROSS ANATOMY
The liver can be divided into the right and left lobes by the middle hepatic vein and a plane between the inferior vena cava and the gallblad-der fossa. Anteriorly, this division is visible by the falciform ligament. Viewed from the under surface, there are also the quadrate lobe in the vicinity of the gallbladder fossa and the caudate lobe which in part encases the vena cava.
There are dual afferent blood supplies in the liver: hepatic artery and portal vein. The hepatic artery (branches from the celiac artery) transports 30 to 40 percent and the portal vein (drains the intestine) transports up to 70 per-cent of the oxygenated blood to the liver. The sinusoids then carry blood from hepatic arteries and portal veins to the terminal hepatic venules, and subsequently through the hepatic vein to the inferior vena cava.
Functionally, the liver is better divided into eight segments based on the blood supply, known as the Couinaud classification, or Cou-inaud scheme (fig. 1-2). The segments are num-bered in Roman numerals I to VIII. Segment I essentially represents the caudate lobe, which drains directly into the inferior vena cava. Seg-ments II to VIII are then numbered in a clockwise manner in a frontal plane beginning superiorly

Tumors of the Liver
2
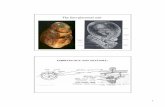
Figure 1-1
FETAL LIVER
Left: The embryonic ductal plate, composed of cytokeratin-rich cells, forms a layer that surrounds the portal area. This immunostain for cytokeratin (CK) 18 shows that the ductal plate is discontinuous and in a few places forms a double layer of cells with small lumina.
Right: Higher magnification of another portal area shows the bile duct forming from the ductal plate, which will eventually disappear as the liver grows.
Figure 1-2
SEGMENTAL ANATOMY OF
THE LIVER
The segments, designated by Ro-man numerals, can be resected sur gically because each is sup-plied by a major branch of the hepatic artery and portal vein and drained by a major tributary of the hepatic vein.
from the left lobe. These segments are bounded by the three main branches of the hepatic veins: the left, middle, and right hepatic veins. Each segment drains into branches of the hepatic veins and then into the inferior vena cava. Overall, seg-ments I to IV represent the functional left lobe, whereas segments V to VIII are considered the functional right lobe. Division of the liver accord-
ing to the Couinaud scheme allows the surgical removal of a single segment, or two or more adjacent segments en bloc, in such a fashion to avoid damaging the remaining segments. The Couinaud scheme also serves as a common ter-minology for communicating among physicians of different disciplines: surgeons, hepatologists, oncologists, radiologists, and pathologists (10).

3
Embryology, Histology, and Anatomy
Figure 1-3
HISTOLOGIC ORGANIZATION OF THE LIVER
Left: Hepatic Architecture. Two views of the architecture of the liver as seen in microscopic sections. The lobules have a central vein at the center and portal spaces at the corners. A cross section of an acinus superimposed on two lobules shows that zone 3 tends to be centrilobular, zone 2 midzonal, and zone 1 periportal, but all three are crescent shaped and may extend into other parts of the classic lobule.
Bottom: Liver Blood Flow. Figure from “Gastro intestinal Tract—Liver Development” from embryology.med.unsw.edu.au.
HISTOLOGIC ORGANIZATION
The liver is structurally organized into pa-renchymal, vascular, bile ductal, and interstitial components (11). Traditionally, the smallest functional unit has been conceptualized as the hepatic lobule or three dimensionally as the hepatic acinus, as defined by Rappaport (12). Most oxygenated blood flows from the terminal branches of portal veins and hepatic arteries in the portal tracts, via the sinusoids to supply the hepatocytes, and finally draining into the terminal branches of the hepatic (cen-tral) veins at the peripheral part of the acinus (11). The acinus can also be conceptualized as a three- dimensional spherical structure with
portal tracts at the center. The spherical area of those hepatocytes surrounding the portal tract is referred to as zone 1, which has the richest oxygenated blood. The area outside of zone 1 is referred to as zone 2. Zone 3 is further out, corresponding to the region surrounding the terminal hepatic vein, where the oxygen con-tent in blood is the lowest (fig. 1-3).
The two-dimensional concept of the lobule, as seen on slides, serves the purpose of histo-logic visualization, but zonal subdivision of the acinus incorporates the physiologic concept into histologic organization and facilitates the recognition of certain liver injury patterns. These patterns include the gradual increasing vulnerability to ischemic and toxic/metabolic

Tumors of the Liver
4
Figure 1-4
HEPATOCYTES AND ENDOTHELIAL CELLS
Left: Normal hepatocytes are polyhedral cells arranged in plates that are one cell thick. They have granular, eosinophilic cytoplasm and usually one nucleus. The sinusoids between the hepatocytes are lined by inconspicuous endothelial cells.
Right: The hepatocytic plates are normally one cell layer in thickness and are separated by the sinusoidal space (reticulin stain).
injuries from zone 1 through zone 2 to zone 3, as well as the distinction between adult nonal-coholic steatohepatitis (zone 3 accentuated) and pediatric nonalcoholic steatohepatitis (zone 1 accentuated) (13–15).
Hepatocytes are formed in plates of one cell in thickness, which can be highlighted by a reticulin stain (fig. 1-4, right). The hepatocytic plates are organized in a radial fashion between the portal tracts and terminal hepatic venules (fig. 1-5), and are separated by sinusoids lined by endothelial cells, with oxygenated blood flowing from the portal tracts to the terminal hepatic venules. The radial alignment is most pronounced in the hepatocyte plates surround-ing the terminal hepatic venules. In normal liver, the portal tracts are composed of at least one hepatic artery branch, one portal vein branch, and one interlobular bile duct (fig. 1-6). The boundary between the portal tracts and the first layer of hepatocytes in the parenchyma is termed the limiting plate.
Bile ductules and canals of Hering are struc-tures at the periphery of the portal tracts and are not conspicuous by hematoxylin and eosin (H&E) stain in normal liver. They are the con-duits between the biliary tree and hepatocyte canaliculi. The canals of Hering drain bile from bile canaliculi into bile ductules, and then into the interlobular bile ducts. Canals of Hering are important for liver regeneration since the hepatic progenitor cells reside here (16,17).
HISTOLOGY
Cells of the Liver
Hepatocytes. Hepatocytes, which originate from endoderm, comprise the primary cell pop-ulation in liver, accounting for 75 to 80 percent of the liver volume (18). They are hexagonal or polyhedral in cross section, and have an eosin-ophilic cytoplasm containing a centrally placed round nucleus; occasional hepatocytes may be binucleatic. In the adult liver, hepatocytes are

5
Embryology, Histology, and Anatomy
Figure 1-7
HEPATOCYTES
Canalicular structures between the hepatocytes are highlighted by polyclonal carcinoembryonic antigen (CEA) due to cross reaction with the glycoprotein on the canalicular surface.
Figure 1-5
HEPATIC ARCHITECTURE
Plates of hepatocytes extend from the portal area (lower left) to the terminal hepatic venule or central vein (upper right). Blood that enters the liver through branches of the portal vein and hepatic artery perfuses the sinusoids and exits through tributaries of the hepatic vein.
Figure 1-6
PORTAL TRACT
A normal portal tract has at least one portal vein, one hepatic artery, and one bile duct. The interlobular bile duct is approximately the same diameter as the hepatic artery and is approximately the same distance as its own diameter away from it.
aligned as anastomosing plates, normally one cell layer in thickness (fig. 1-4). The hepatocytic plates are separated by sinusoids, in which the oxygenated blood flows from the portal tracts to the terminal hepatic venules.
Bile is produced exclusively by hepatocytes and secreted into the bile canaliculi, which are gutter-like structures between two or three adja-cent hepatocytes in the hepatocytic plates (11). The canalicular surface is lined by glycoprotein I, which cross reacts with polyclonal carcinoembry-onic antigen (CEA), an immunohistochemical marker specific for hepatocytic differentiation (fig. 1-7), although its sensitivity is not optimal and its interpretation requires experience. Can-alicular staining is also observed using a CD10 immunostain (19). Immunohistochemical stains for cytokeratin (CK) 8 and 18 are positive in he-patocytes, as is CAM5.2 (20). Since the hepatocytes are rich in urea cycle proteins, antibodies against some urea cycle enzymes have been used widely as hepatocytic markers, such as hepatocyte paraf-fin-1 (HepPar1) (21) and arginase-1 (22). Various cytoplasmic contents, pigments, and materials are also present within the hepatocytes, including fat, glycogen, bile, lipofuscin, and hemosiderin. However, they are not always seen nor are they absolutely specific for hepatocytic differentiation.

Tumors of the Liver
6
Figure 1-9
PERIBILIARY GLANDS
Small glands in the walls of large bile ducts are composed of small acini of cuboidal cells with clear cytoplasm.
Biliary Epithelial Cells. The lining epithe-lial cells of the septal or larger intrahepatic bile ducts are tall columnar to cuboidal, while those of the interlobular bile ducts are typically cuboidal (fig. 1-8). These cells are encased by a layer of periodic acid–Schiff (PAS)-positive base-ment membrane and are further surrounded by connective tissue of variable amount, depend-ing on the size of the bile ducts. The interlobular bile duct and hepatic artery are typically adja-cent to each other, with a similar diameter and a distance apart approximately the same as their own diameters. The bile ducts are connected to the bile canaliculi via the canals of Hering. On cross section, the configuration of the bile duct appears as a chain of pearls (fig. 1-8). Bile ductal cells express CK7 and CK19, reflected by immunohistochemical staining. Like the hepatocytes, they also express CK8 and CK18.
Peribiliary Gland Cells. Peribiliary glands are small accessory glands of the bile ducts in liver. They communicate with the lumens of the intrahepatic and extrahepatic large bile ducts via specialized conduits (7,23,24), densely populated
in the hilar bile ducts, cystic duct, and periamp-ullary areas (24–28). Peribiliary glands are com-posed of glandular and branched tubuloalveolar structures that form small acini that surround the intrahepatic and extrahepatic large bile ducts. The glandular cells are cuboidal, clear (fig. 1-9), and seromucinous in nature, secreting lactoferrin and lysozyme (26).
Ductular Cells. Bile ductules are also known as cholangioles. The terms bile ductules and ca-nals of Hering are sometimes used interchange-ably, however, they represent two different physiologic and histologic structures. The canals of Hering are the physiologic link between the biliary tree and hepatocyte canaliculi (29). They are lined partially by cholangiocytes and par-tially by hepatocytes. They continue into bile ductules, which represent the conduits between canals of Hering and the interlobular bile ducts. Bile ducts are lined entirely by cholangiocytes.
The bile ductules are situated at the edge of the portal tracts. They may also penetrate the limiting plate so they not only have an intra-portal segment, but also have an intralobular
Figure 1-8
INTERLOBULAR BILE DUCTS
Lining cells of the interlobular bile ducts are cuboidal and form a circular structure in cross section. Their nuclei are round and regular, without prominent nucleoli.

7
Embryology, Histology, and Anatomy
Figure 1-10
BILE DUCTULES
A: The ductules, or cholangioles, are normally incon-spicuous, but they become apparent and increased in number in reaction to various pathologic conditions. In this case of primary sclerosing cholangitis, the ductules are apparent and increased in number in reaction to biliary obstruction.
B: Immunohistochemical staining for CK19 highlights the reacting ductules.
C: Ductular reaction in a case of submassive hepatocellular necrosis due to acetaminophen overdose. The ductules reflect a regenerative phenomenon in response to liver injury.
segment (11,29). Unlike the interlobular bile duct, the bile ductules are not accompanied by a hepatic artery branch (11). Their lumens are also smaller than those of interlobular bile ducts (29). The lining cells of the canals of Her-ing stain for CK7, CK19, and the hepatic stem cell marker NCAM/CD56, demonstrating the scattered presence of hepatic progenitor cells in these structures (16,17).
Ductular reaction is a phenomenon seen in diseased liver, either acute or chronic disease (fig. 1-10). It is a reaction of ductular phenotype, but not necessarily of ductular origin (29), as it may arise from proliferation of preexisting cholan-giocytes, progenitor cells (either local and/or
circulating cells such as bone marrow-derived), or biliary metaplasia of hepatocytes (rare) (29).
Endothelial Cells. The endothelial cells lining the portal veins, hepatic arteries, and terminal hepatic venules share the same prop-erties and immunophenotype as the endothelial cells lining the arteries, veins, and capillaries in other organs. Their single layer of flattened endothelial cells is attached above a basement membrane and lines the vascular spaces of these vessels. They are immunoreactive to antibodies against CD31, CD34, and factor VIII-related antigen (von Willebrand factor).
In contrast, endothelial cells lining the sinu-soidal spaces do not have a basement membrane

Tumors of the Liver
8
Figure 1-11
SINUSOIDAL ENDOTHELIAL CELLS
The endothelial cells lining the sinu soidal spaces are inconspicuous (arrows).
Figure 1-12
KUPFFER CELLS
Kupffer cells enlarge and become apparent when they engulf and phagocytose debris resulting from liver injury. They are highlighted by a CD68 immunostain.
(fig. 1-11). In normal liver, the sinusoidal spaces are fenestrated and are lined by endothelial cells that are immunohistochemically nonreactive to CD31 or CD34. The fenestration allows plasma to be transported between the sinusoidal spaces and the hepatocytes underneath the subendo-thelial space of Disse. When there is neoplastic change of the hepatocytes, the fenestration is decreased, and the sinusoidal endothelial cells become reactive to CD31 or CD34, a phenom-enon known as capillarization of the sinusoids. These changes are also observed in preneoplastic lesions, i.e., dysplastic nodules (30–32). Capillar-ization also occurs in livers with chronic injury or cirrhosis, beginning at the periportal region and extending to the hepatic lobules, although the changes are not as significant as in neoplasia.
Kupffer Cells. Kupffer cells are specialized macrophages of the mononuclear phagocyte system that line the walls of the sinusoidal spaces. Normally they are not prominent, but
enlarge and become noticeable when they en-gulf and phagocytose debris resulting from liver injury (fig. 1-12). Hemosiderin pigments may be deposited in Kupffer cells, due to secondary iron overload, or more rarely, hereditary hemo-chromatosis due to mutation in the SLC40A1/ferroportin 1 gene (33). Lipofuscin pigment may also appear in Kupffer cells.
Kupffer cells are variably PAS positive and diastase resistant since they contain many ly-sosomes. They are also positive for macrophage markers such as CD68 (KP1).
Hepatic Stellate Cells. The compartment between the hepatocyte cytoplasmic membrane and the sinusoidal space, underneath the fenes-trated sinusoidal endothelial cells, is the space of Disse, where hepatic stellate cells reside. A number of synonyms were previously used for these cells, such as Ito cells, fat-storing cells, perisinusoidal cell lipocytes, and parasinusoidal cells, but the preferred nomenclature by the

9
Embryology, Histology, and Anatomy
Figure 1-13
HEPATIC STELLATE CELLS
Left: Hepatic stellate cells are identified with an immunostain for smooth muscle actin.Right: The hepatic stellate cells become conspicuous in hepatotoxicity due to hypervitaminosis A, which shows bubbly
cells due to the deposition of fat in the cytoplasm, with hyperchromatic and scalloped nuclei.
consensus of investigators studying this cell is hepatic stellate cell (34).
Within the subendothelial spaces of normal liver, these cells store vitamin A (retinoid) drop-lets in their cytoplasm (35). In their quiescent phase, they are not conspicuous in formalin-fixed normal liver, but may become discernible by im-munohistochemistry using anti-smooth muscle actin (fig. 1-13, left) or anti-desmin antibodies. Hepatic stellate cells are also prominent during hepatotoxicity associated with hypervitaminosis A, becoming bubbly cells due to the deposition of fat in the cytoplasm, with hyperchromatic and scalloped nuclei (fig. 1-13, right).
Hepatic stellate cells are responsible for he-patic fibrogenesis. They can transform into my-ofibroblasts when they are activated in response to chronic liver disease with activated TGF-b and other signaling pathways. This phenotypic change triggers a series of accumulation as well
as inhibition of the breakdown of matrix pro-teins, hence leading to liver fibrosis (36).
Pit Cells. Another cell residing in the space of Disse is the pit cell. Pit cells are large granule- containing lymphoid cells possessing natural killer cell properties that are liver specific (37). Pit cells are not readily identifiable under light microscopy, but have been characterized by electron microscopy as cells containing mul-tivesicular body-related dense granules and rod-cored vesicles (38).
Inflammatory Cells. In normal liver, in-flammatory cells are inconspicuous, except for occasional lymphocytes, Kupffer cells, and neutrophils in the sinusoids. The portal tracts have few lymphocytes and macrophages, and their number appears to correlate with age, likely reflecting a remnant response to previous injuries in the liver.

Tumors of the Liver
10
REFERENCES
1. Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embry-onic liver bud and ventral foregut tissues. Dev Biol 2005;280:87-99.
2. Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol 2012;56:1159-1170.
3. Houssaint E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interac-tions in hepatocyte differentiation. Cell Differ 1980;9:269-279.
4. Tan J, Hytiroglou P, Wieczorek R, et al. Immu-nohistochemical evidence for hepatic progenitor cells in liver diseases. Liver 2002;22:365-373.
5. Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628-635.
6. Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 2009;137:62-79.
7. Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Mi-crostructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech 1997;38:552-570.
8. Libbrecht L, Cassiman D, Desmet V, Roskams T. Expression of neural cell adhesion molecule in human liver development and in congenital and acquired liver diseases. Histochem Cell Biol 2001;116:233-239.
9. Fabris L, Cadamuro M, Libbrecht L, et al. Epi-thelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology 2008;47:719-728.
10. Majno P, Mentha G, Toso C, Morel P, Peitgen HO, Fasel JH. Anatomy of the liver: an outline with three levels of complexity—a further step towards tailored territorial liver resections. J Hepatol 2014;60:654-662. 10. Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol 2016;13:301-315.
11. Suriawinata A, Thung S. Liver. In: Mills SE, ed. Histology for pathologists, 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2012:733-758.
12. Rappaport AM. The structural and functional unit in the human liver (liver acinus). Anat Rec 1958;130:673-689.
13. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-1321.
14. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641-649.
15. Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 2014;147:754-764.
16. Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in hu-mans. Hepatology 1999;30:1425-1433.
17. Turner R, Lozoya O, Wang Y, et al. Human he-patic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-1045.
18. Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol 2016;13:301-315.
19. Loke SL, Leung CY, Chiu KY, Yau WL, Cheung KN, Ma L. Localisation of CD10 to biliary cana-liculi by immunoelectron microscopical exam-ination. J Clin Pathol 1990;43:654-656.
20. van Eyken P, Sciot R, van Damme B, de Wolf-Peeters C, Desmet VJ. Keratin immunohisto-chemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histo-pathol 1987;412:63-72.
21. Butler SL, Dong H, Cardona D, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest 2008;88:78-88.
22. Yan BC, Gong C, Song J, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol 2010;34:1147-1154.
23. Terada T, Nakanuma Y, Ohta G. Glandular ele-ments around the intrahepatic bile ducts in man; their morphology and distribution in normal livers. Liver 1987;7:1-8.
24. Igarashi S, Sato Y, Ren XS, Harada K, Sasaki M, Nakanuma Y. Participation of peribiliary glands in biliary tract pathophysiologies. World J Hepa-tol 2013;5:425-432.
25. Tsuneyama K, Kono N, Yamashiro M, et al. Ab-errant expression of stem cell factor on biliary epithelial cells and peribiliary infiltration of c-kit-expressing mast cells in hepatolithiasis and primary sclerosing cholangitis: a possi-ble contribution to bile duct fibrosis. J Pathol 1999;189:609-614.
26. Nakanuma Y, Zen Y, Portman B. Diseases of the bile ducts. In: MacSween RN, Burt AD, Portman B, Ferrell LE, eds. MacSween’s pathology of the liver. Edinburg: Churchill Livingstone/Elsevier; 2012:491-562.

11
Embryology, Histology, and Anatomy
27. Terada T, Nakanuma Y, Ohta G. Glandular ele-ments around the intrahepatic bile ducts in man; their morphology and distribution in normal livers. Liver1987;7:1-8.
28. Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol 2011;197:1111-1120.
29. Roskams TA, Theise ND, Balabaud C, et al. No-menclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739-1745.
30. Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma. An immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med 1995;119: 173-178.
31. Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and si-nusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol 1998;22:656-662.
32. Bedossa P, Hytiroglou P, Yeh M. Vascular Dis-orders. In: Burt A, Ferrell LD, Hubscher S, eds. MacSween’s pathology of the liver, 7th ed: Phil-adelphia: Elsvier; 2018:636-672.
33. Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis 2004;32:131-138.
34. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-172.
35. Wake K. “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat 1971;132:429-462.
36. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425-456.
37. Wisse E, Luo D, Vermijlen D, Kanellopoulou C, De Zanger R, Braet F. On the function of pit cells, the liver-specific natural killer cells. Semin Liver Di. 1997;17:265-286.
38. Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphol-ogy and function. Med Electron Microsc 2004; 37:29-36.