1 © 2006 Brooks/Cole - Thomson Electrolysis Using electrical energy to produce chemical change. Sn...
-
Upload
ashley-warner -
Category
Documents
-
view
219 -
download
0
description
Transcript of 1 © 2006 Brooks/Cole - Thomson Electrolysis Using electrical energy to produce chemical change. Sn...
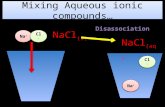
1 2006 Brooks/Cole - Thomson Electrolysis Using electrical energy to produce chemical change. Sn 2+ (aq) + 2 Cl - (aq) ---> Sn(s) + Cl 2 (g) 2 2006 Brooks/Cole - Thomson Electrolysis Electric Energy ---> Chemical Change Electrolysis of molten NaCl. Electrolysis of molten NaCl. Here a battery pumps electrons from Cl - to Na +. Here a battery pumps electrons from Cl - to Na +. NOTE: Polarity of electrodes is reversed from batteries. NOTE: Polarity of electrodes is reversed from batteries. 3 2006 Brooks/Cole - Thomson Electrolysis of Molten NaCl Figure 20.18 4 2006 Brooks/Cole - Thomson Electrolysis of Molten NaCl Anode (+) 2 Cl - ---> Cl 2 (g) + 2e- Cathode (-) Na + + e- ---> Na E o for cell (in water) = V (in water) External energy needed because E o is (-). 5 2006 Brooks/Cole - Thomson Electrolysis of Aqueous NaI Anode (+): 2 I - ---> I 2 (g) + 2e- Cathode (-): 2 H 2 O + 2e- ---> H OH - E o for cell = V 6 2006 Brooks/Cole - Thomson Electrolysis of Aqueous CuCl 2 Anode (+) 2 Cl - ---> Cl 2 (g) + 2e- Cathode (-) Cu e- ---> Cu E o for cell = V Note that Cu 2+ is more easily reduced than either H 2 O or Na +. 7 2006 Brooks/Cole - Thomson Michael Faraday Originated the terms anode, cathode, anion, cation, electrode. Discoverer of electrolysiselectrolysis magnetic props. of mattermagnetic props. of matter electromagnetic inductionelectromagnetic induction benzene and other organic chemicalsbenzene and other organic chemicals Was a popular lecturer. 8 2006 Brooks/Cole - Thomson Quantitative Aspects of Electrochemistry Consider electrolysis of aqueous silver ion. Ag + (aq) + e- ---> Ag(s) 1 mol e----> 1 mol Ag If we could measure the moles of e-, we could know the quantity of Ag formed. But how to measure moles of e-? 9 2006 Brooks/Cole - Thomson But how is charge related to moles of electrons? Quantitative Aspects of Electrochemistry = 96,500 C/mol e- = 1 Faraday Michael Faraday 10 2006 Brooks/Cole - Thomson Quantitative Aspects of Electrochemistry 1.50 amps flow through a Ag + (aq) solution for 15.0 min. What mass of Ag metal is deposited? Solution (a)Calc. charge Charge (C) = current (A) x time (t) = (1.5 amps)(15.0 min)(60 s/min) = 1350 C 11 2006 Brooks/Cole - Thomson Quantitative Aspects of Electrochemistry Solution (a)Charge = 1350 C (b)Calculate moles of e- used 1.50 amps flow through a Ag + (aq) solution for 15.0 min. What mass of Ag metal is deposited? (c)Calc. quantity of Ag 12 2006 Brooks/Cole - Thomson Quantitative Aspects of Electrochemistry The anode reaction in a lead storage battery is Pb(s) + HSO 4 - (aq) ---> PbSO 4 (s) + H + (aq) + 2e- If a battery delivers 1.50 amp, and you have 454 g of Pb, how long will the battery last? Solution a)454 g Pb = 2.19 mol Pb b)Calculate moles of e- c)Calculate charge 4.38 mol e- 96,500 C/mol e- = 423,000 C 4.38 mol e- 96,500 C/mol e- = 423,000 C 13 2006 Brooks/Cole - Thomson Quantitative Aspects of Electrochemistry The anode reaction in a lead storage battery is Pb(s) + HSO 4 - (aq) ---> PbSO 4 (s) + H + (aq) + 2e- If a battery delivers 1.50 amp, and you have 454 g of Pb, how long will the battery last? Solution a)454 g Pb = 2.19 mol Pb b)Mol of e- = 4.38 mol c)Charge = 423,000 C About 78 hours d)Calculate time











![Aula #23 · AgCl (s) Ag+ (aq) + Cl-(aq) Ksp = [Ag +][Cl K sp is the solubility product constant MgF 2 (s) Mg2+ (aq) + 2F-(aq) Ksp = [Mg 2+][F]2 Ag 2 CO 3 (s) 2Ag+ (aq) + CO3 2-(aq)](https://static.fdocuments.in/doc/165x107/5f08237a7e708231d42087a7/aula-23-agcl-s-ag-aq-cl-aq-ksp-ag-cl-k-sp-is-the-solubility-product.jpg)

![In aqueous solution… In aqueous solution… HCl (aq) H + (aq) + Cl - (aq) Acids Acids increase hydrogen ion concentration [H + ] Courtesy Christy Johannesson.](https://static.fdocuments.in/doc/165x107/56649f345503460f94c51169/in-aqueous-solution-in-aqueous-solution-hcl-aq-h-aq-cl-.jpg)



![Solubility Equilibria 16.6 AgCl (s) Ag + (aq) + Cl - (aq) K sp = [Ag + ][Cl - ]K sp is the solubility product constant MgF 2 (s) Mg 2+ (aq) + 2F - (aq)](https://static.fdocuments.in/doc/165x107/56649f355503460f94c53668/solubility-equilibria-166-agcl-s-ag-aq-cl-aq-k-sp-ag-cl.jpg)

