Wet Cell: A Cell containing a liquid electrolyte Electrode: An electrical conductor....
-
Upload
maurice-porter -
Category
Documents
-
view
225 -
download
0
Transcript of Wet Cell: A Cell containing a liquid electrolyte Electrode: An electrical conductor....

THE COIN BATTERY


So… What did we really do here?
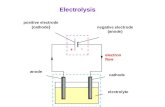
Wet Cell: A Cell containing a liquid electrolyte
Electrode: An electrical conductor. Electrochemical reactions occur on the surface of an electrode.
Electrolyte: An electrically conductive substance.
Electricity: The flow of electrons. Electric Circuit: A closed loop of
conductive material that allows for the flow of electrons.

The pennies release a positive charge
The nickels release a negative charge.
Your battery has a negative and a positive end just like one you might buy at a store.

What happened when you only had one finger on the battery?
What happened when you held it between your index fingers?

In a battery electrons flow from the negative terminus to the positive one. Nickel to penny.
Each series of 2 coins in your battery is it’s own wet cell, and has it’s own small charge.
The charge gets big enough to detect when we link many of these small cells together.
That alone is not enough though! Your battery does not conduct any electricity until you complete the electrical circuit.

When you held the battery between your two index
fingers you completed the
electrical circuit, allowing electrons
to flow!!!

The Baghdad Battery

The Baghdad Battery
The Baghdad battery was discovered just outside of
Baghdad in 1936. It is believed to be about 2000 years old, making it the first known battery. Some scientists believe that ancient people used this battery for
plating gold onto silver objects. Others say that it may have been
used for medicinal purposes.

The Baghdad Battery

Cross-Section of the Baghdad Battery

How it works
The battery would have been filled with an acidic liquid, likely vinegar or fermented grape juice.
This acidic solution would allowed the flow of electrons from the copper tube to the iron rod when the two electrodes are connected by a wire to complete the circuit.
This battery is capable of producing between 1.5-2 Volts of electricity, which is not a lot. It is believed that many of these cells were linked to produce a charge that would be large enough to be useful.

References
Coin battery picturehttp://activitymama.wordpress.com/2009/04/19/coin-batteries/Positive-negative battery picturehttp://itp.nyu.edu/physcomp/uploads/battery_parallel.jpgBaghdad battery picture http://www.skepticworld.com/ancient-artifacts/images/baghdad_battery_2.jpgBaghdad battery Cross-sectionhttp://www.smith.edu/hsc/museum/ancient_inventions/battery1a.JPGBaghdad Battery labelled diagramhttp://www.watsonsupply.com/charley/batteries/images/battery.gifInfo on the Baghdad Batteryhttp://en.wikipedia.org/wiki/Baghdad_Batteryhttp://theunexplainedmysteries.com/egyptian-lamp.htmlInfo on the coin batteryhttp://activitymama.wordpress.com/2009/04/19/coin-batteries/Connolly, Sean, Wholly Irresponsible Science, London, England, Icon Books
Ltd.